Using a Custom App to Characterize Calcium Transients in Patient-derived Cardiomyocytes
Organization
Cara Hawey
Hébert Lab
Department of Pharmacology and Therapeutics
McGill University, Montréal, Quebec
Problem
Cara was studying calcium dysregulation in cells derived from patients with dilated cardiomyopathy, and dealing with large datasets. Initial analysis methods had proven very time-consuming and so she approached OriginLab in hopes of automating some of her analysis work.
Solution
Cara worked with OriginLab to develop an App that helps her to easily gather and analyze data to determine the features of her calcium transients.
“
Origin has given me a way to effectively analyze my data to alleviate the time burden associated with analyzing large data sets.
”
Cara Hawey is currently a Master's student in Dr. Terry Hébert's Lab, Department of Pharmacology and Therapeutics, McGill University in Montréal, Quebec. Her research focuses on use of patient-derived induced pluripotent stem cell cardiomyocytes (iPSC-CMs) to characterize dilated cardiomyopathies.
Stem cells are widely known in the context of embryo development; however, stem cells can also be derived from adult humans. Cells from tissue such as skin or blood can be taken from a person and genetically reprogrammed to stem cells, which are then able to become any cell type. These cells are known as induced pluripotent stem cells (iPSCs). iPSCs are a powerful tool in personalized disease modeling and can be used to develop “disease in a dish” models. Simple models of heart diseases can be developed by turning iPSCs into cardiomyocytes. Cardiomyocytes are specialized cells within the myocardium that are responsible for the contractile function of the heart. This research involves use of patient-derived cardiomyocytes to study dilated cardiomyopathies (DCM), a group of diseases that result in impaired contractility of the heart muscle. In particular, Cara is looking at how calcium dysregulation may play a role in disease and how it may relate to impaired contractility.
Cara's research generates large amounts of data and she had found her early analysis work to be very time-consuming. Subsequently, she had contacted OriginLab out of a desire to automate her analyses and make her workflow more manageable. As she initially explained her problem: "I use a genetically-encoded fluorescent biosensor to track calcium transients of cardiomyocytes on a single-cell level. I extract the fluorescence data from TIFF stacks to measure parameters such as tau decay, time to peak, amplitude, and wave duration. I am currently using Excel-based methods of data analysis but would like to develop a more time-efficient and simple way to gather data from calcium transients." Working from sample datasets, Dr. Yiming Chen of OriginLab's technical support and applications development group worked with Cara to build a custom application that met her needs.
We asked Cara to tell us a bit about the mechanics of her research and how she is using the "Transient Analysis" custom application to analyze her data:
"To monitor calcium transients in iPSC-CMs at the single cell level, I am using a genetically-encoded fluorescent biosensor called RGECO-TnT that localizes to the myofilament (1) . The myofilament is the contractile unit of the cardiomyocyte and is home to the troponin complex. Calcium binds the troponin complex to initiate contractions of cardiomyocytes. The fluorescent protein RGECO was bound to a member of the troponin complex to make RGECO-TnT. RGECO-TnT is an intensiometric biosensor that fluoresces in response to calcium binding at the troponin complex. This biosensor can be used to monitor calcium handling at the myofilament level in single cells."
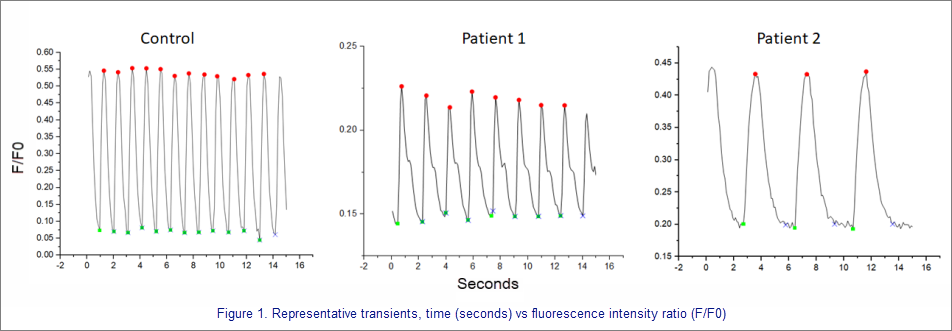
"Data may be collected using fluorescent microscopes, such as the Zeiss Axio Observer Fully Automated Inverted microscope. The iPSC cardiomyocytes from patients with DCM as well as healthy controls are plated onto black-walled 96 well plates with optical bottoms. The cells are kept in a gas- and temperature-controlled chamber on the microscope. Using a red fluorescent filter and a 20X objective, image stacks of spontaneous calcium transients are recorded for a duration of 15 seconds."
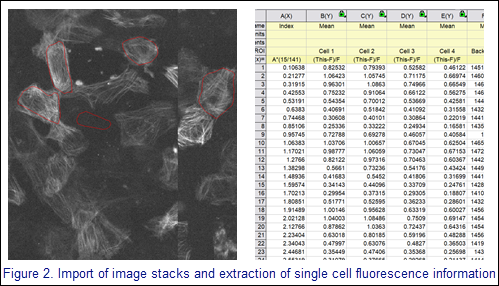
Once the image stacks are created, Cara imports the multi-frame images into Origin matrices. She then uses the Region Tool to select individual cells as well as sample background fluorescence and labels them. Intensity data are extracted for all matrix regions-of-interest (ROIs), and cell fluorescence is normalized to the background fluorescence. The frame number is converted to a time within the 15 second sequence.
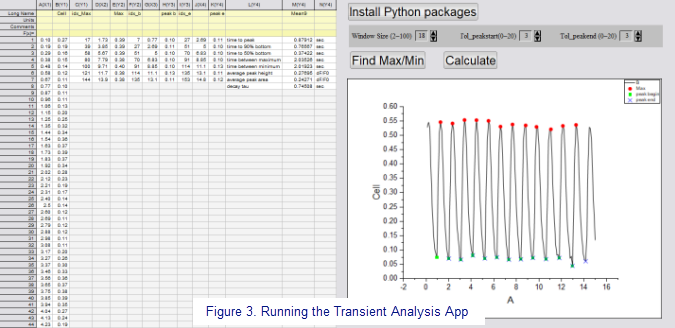
"The Calcium Transient Analysis App (co-developed with OriginLab) is then run to determine features of the calcium transients including time-to-peak, time to 50% baseline, time to 90% baseline, time between maximum, transient duration, peak height, and area under the curve. Monoexponential curve fitting is used to determine decay tau. These features are determined for each individual peak and averaged to produce one value per cell. Results for every cell in an image stack are provided in a workbook summary tab. These features will be used to compare patients to patients as well as patients to controls to determine how calcium is dysregulated at the myofilament. This assay can also be used to determine how calcium handling may become dysregulated over time in response to chronic insult."
“
The Calcium Transient Analysis App provides a very user-friendly and straight-forward approach to data graphing and statistical analysis.
”
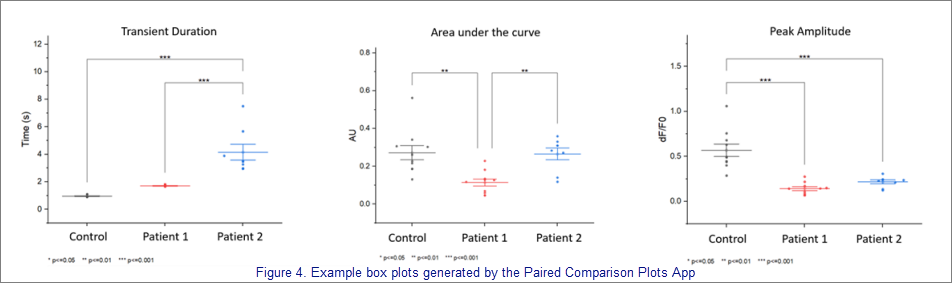
Cara notes that the Calcium Transient Analysis App allows window size to be adjusted to better determine the number of peaks. Peak start and peak end can be adjusted to identify the beginning and end of peaks more accurately, if necessary. "After every adjustment I find Max/Min and once peaks are accurately identified, I run 'Calculate.' The summary tab is updated and results for cells are compiled to make comparisons accordingly (i.e., patient vs control, patient over time in response to chronic treatment)." Cara then uses OriginLab's Paired Comparisons Plot App to graphically compare groups and complete statistical analysis.
Cara tells us that she plans to use the custom Calcium Transient Analysis App for all of her calcium analysis. "Additional applications of this assay can investigate potential therapeutics and draw associations between calcium handling and other features underlying disease such as dysregulated signalling pathways and phenotypes such as impaired contractility or disorganized sarcomeres."
In addition, she expects that others in her lab will also find the App to be useful. "The Calcium Transient Analysis app can be used to analyze any transient such as voltage-related transients, to track electrophysiological properties of iPSC-CMs derived from DCM patients and healthy controls."
References
(1) Measurement of Myofilament-Localized Calcium Dynamics in Adult Cardiomyocytes and the Effect of Hypertrophic Cardiomyopathy Mutations